來源:Physiological Reports ISSN 2051-817X 作者:Steven S. Laurie, Gianmarco Vizzeri, Giovanni Taibbi, Connor R. Ferguson, Xiao Hu, Stuart M. C. Lee, Robert Ploutz-Snyder, Scott M. Smith, Sara R. Zwart & Michael B. Stenger 由德力凱代理商MULTIGON INDUSTRIES.INC.提供設(shè)備。
Many astronauts experience ocular structural and functional changes during long-duration spaceflight, including choroidal folds, optic disc edema, globe flattening, optic nerve sheath diameter (ONSD) distension, retinal nerve fiber layer thickening, and decreased visual acuity. The leading hypothesis suggests that weightlessness-induced cephalad fluid shifts increase intracranial pressure (ICP), which contributes to the ocular structural changes, but elevated ambient CO2 levels on the International Space Station may also be a factor. We used the spaceflight analog of 6° head-down tilt (HDT) to investigate possible mechanisms for ocular changes in eight male subjects during three 1-h conditions: Seated, HDT, and HDT with 1% inspired CO2 (HDT + CO2). Noninvasive ICP, intraocular pressure (IOP), translaminar pressure difference (TLPD = IOP-ICP), cerebral and ocular ultrasound, and optical coherence tomography (OCT) scans of the macula and the optic disc were obtained. Analysis of one-carbon pathway genetics previously associated with spaceflight- induced ocular changes was conducted. Relative to Seated, IOP and ICP increased and TLPD decreased during HDT. During HDT + CO2 IOP increased relative to HDT, but there was no significant difference in TLPD between the HDT conditions. ONSD and subfoveal choroidal thickness increased during HDT relative to Seated, but there was no difference between HDT and HDT + CO2. Visual acuity and ocular structures assessed with OCT imaging did not change across conditions. Genetic polymorphisms were associated with differences in IOP, ICP, and end-tidal PCO2. In conclusion, acute exposure to mild hypercapnia during HDT did not augment cardiovascular outcomes, ICP, or TLPD relative to the HDT condition.
Introduction
Astronauts completing long-duration spaceflight of up to ~6 months have developed ocular structural and functional changes including choroidal folds, optic disc edema, globe flattening, optic nerve sheath distension, retinal nerve fiber layer (RNFL) thickening, and visualacuity decrements (Mader et al. 2011; Alexander et al. 2012), although not all symptoms have developed in all crewmembers. The initial report of these findings in 2011 suggested that postflight lumbar puncture opening pressures were slightly elevated based on measurements in five of six astronauts who developed optic disc edema, although no preflight measurements were available forcomparison (Mader et al. 2011). Thus, it was hypothesized that increased intracranial pressure (ICP) may be responsible for the above ocular findings, perhaps secondary to the weightlessness-induced headward fluid shift, although ICP has never been measured in human subjects during spaceflight. The spaceflight analog of 6° head-down tilt (HDT) causes a headward fluid shift similar to that which occurs during spaceflight (Blomqvist et al. 1980; Lathers et al. 1990; Pavy-Le Traon et al. 2007), but frank optic disc edema, choroidal folds, or decreased near visual acuity have not developed during prolonged bed rest (Taibbi et al. 2013, 2014, 2016), suggesting additional factors from the spaceflight environment may be necessary for ocular changes to develop. Another factor hypothesized to contribute to the development of ocular changes is exposure to the mildly elevated ambient partial pressure of carbon dioxide (PCO2) that occurs on the International Space Station (ISS) (Law et al. 2010, 2014). Elevated arterial CO2 is a strong vasodilator, and from 2001 to 2012, the range of mean 24-h ambient PCO2 on the ISS was 1.0–5.8 mmHg, with a range in peak 24-h levels of 1.2– 8.3 mmHg. The astronauts described in the first case report of ocular changes flew long-duration missions to the ISS during this period (Mader et al. 2011). Since 2010, efforts have been made to keep the average 24-h PCO2 below 4 mmHg (Law et al. 2014), yet ocular structural and functional findings continue to develop. While these low levels are unlikely to cause symptoms of CO2 toxicity in normal ambulatory subjects on the ground, correlations between 7-day average CO2 levels on the ISS and the predicted probability of headaches suggest thatweightlessness may increase the sensitivity to mild elevations in CO2 (Law et al. 2014). Thus, the combination of a headward fluid shift with mild elevations in CO2 may provide a synergistic stimulus that increases cerebrovascular blood flow and elevates ICP (Sofronova et al. 2015), resulting in an imbalance of fluid pressures at the optic disc and decreases the translaminar pressure difference (TLPD, intraocular pressure [IOP] - ICP). Prolonged exposure to a lowered TLPD may be a factor in developing the ocular structural changes observed during spaceflight. While the etiology of spaceflight-induced ocular changes remains unknown, no studies have linked mild hypercapnia in combination with a headward fluid shift with physiological responses that, if maintained for three to six months, could produce ocular structural and functional changes. Given that not all astronauts develop ocular changes and that individual variability exists in symptom severity, it is likely that some predisposing factors confer greater susceptibility to developing ocular changes in certain astronauts. Genetic differences in the one-carbonmetabolic pathway had been hypothesized to be associated with the development of ophthalmologic changes during spaceflight (Zwart et al. 2012), and recent evidence documents an association between single-nucleotide polymorphisms (SNPs) involved in one-carbon metabolism and choroidal folds, optic disc edema, and cottonwool spots (Zwart et al. 2016). Specifically, of the 49 astronauts studied, the minor G allele on the MTRR 66 gene was more prevalent among those that developed choroidal folds or cotton wool spots, whereas the major C allele for gene SHMT1 1420 was associated with optic disc edema. Furthermore, block regression modeling showed that genetics (the number of risk alleles for each gene) and B-vitamin status were significant predictors of choroidal folds and the degree of visual acuity changes after flight (Zwart et al. 2016). The purpose of this study was to determine whether acute exposure to mild hypercapnia combined with a cephalad fluid shift induced by HDT would increase cerebral or ocular blood flow, result in an increase in ICP, a reduction in TLPD, and a mild accumulation of fluid at the optic nerve. Given that evidence suggests certain individuals may be more susceptible to ocular changes during spaceflight, we also investigated genetic and biochemical factors associated with one-carbon metabolism to determine if individuals with similar risk alleles demonstrated greater responses to mild hypercapnia than those with fewer alleles.
Materials and Methods
Subjects
The NASA Johnson Space Center Institutional Review Board reviewed and approved this human subject protocol and all methods adhered to the principles outlined in the Declaration of Helsinki. After completing a modified Air Force Class III physical exam and being cleared by the NASA Test Subject Screening facility, eight male subjects provided their written informed consent to participate. Subjects were 35 year old (range: 25–49 year), 181 cm tall (range: 170–193 cm), and weighed 86 kg (range: 73–95 kg).
Study protocol
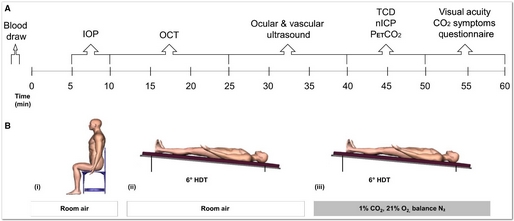
All subjects reported to the NASA Johnson Space Center Cardiovascular & Vision Laboratory for a single visit between 0700 and 0800 for a fasted blood draw. Subjects were fitted with a facemask to control inspired gases and for the sampling of expired CO2, a continuous finger blood pressure monitor, 3-lead ECG, and with a headband holding the transcranial Doppler probe. These data were recorded continuously using a data acquisition system (Notochord) for later offline analysis. Thereafter, subjects were studied for 60 min each in the following conditions: (A) seated upright breathing room air (Seated); (B) 6° HDT breathing room air (HDT); and (C) 6° HDT breathing 1% CO2, 21% O2, balance N2 (HDT + CO2). All subjects completed the Seated condition first, but the order of the two HDT conditions, HDT and HDT + CO2, was randomized and balanced. Each condition began with a 5 min stabilization period before data were collected in the following order: ocular tonometry, optical coherence tomography (OCT), ocular and cardiovascular ultrasound, partial pressure of end-tidal CO2 (PETCO2), near visual acuity, and completion of a CO2 symptom questionnaire (Fig. 1).
Ocular tonometry
An Icare Pro (Icare, Espoo, Finland) rebound tonometer was used to measure intraocular pressure (IOP). Data collected using this instrument has shown good agreement with data obtained with the Goldmann applanation tonometer, the clinical gold standard for IOP measurement (Moreno-Monta~n_es et al. 2015). A trained technician obtained three successive IOP measurements per eye starting with the right eye. Each of the three measurements was the result of six successive trials, of which the highest and lowest values were dropped and the remaining four were averaged by the Icare device.
Optical coherence tomography
Spectralis HRA + OCT (Heidelberg Engineering, Heidelberg, Germany) was used to obtain bilateral OCT scans in High-Resolution mode. The scanning head was mounted on a surgical arm to obtain OCT scans in the seated and head-down tilt conditions. The left eye of each subject was scanned first, followed by the right eye. In each eye, the AutoRescan feature was used to anatomically place followup scans in the same location as the baseline (Seated) condition. The built-in Anatomic Positioning System (APS) was used to obtain optic disc scans aligned to the fovea-to- Bruch’s membrane opening (BMO) center axis to adjust for head-tilting and ocular cyclotorsion across consecutive scans from the same eye and ensure consistent sector analysis over time. Specifically, the radial 24-line scanning pattern aligned to the BMO center was used to obtain the average BMO-minimum rim width (BMO-MRW), defined as the shortest linear distance between the BMO and the internal limiting membrane (Povazay et al. 2007; Chen 2009; Chauhan and Burgoyne 2013). The circular scanning pattern, placed evenly around the BMO center, was used to obtain the average RNFL thickness. The volume scanning pattern, centered to the fovea (25 B-scans over a 20°920° area), was used to obtain macular thickness. The Enhanced Depth Imaging line scanning pattern, aligned to the fovea-to-BMO center axis, was used to measure choroidal thickness. Choroidal thickness was calculated as the distance between Bruch’s membrane, as automatically delineated by Spectralis OCT + HRA software (Heidelberg Eye Explorer, v1.9.10), and the choroidal- scleral border, as manually delineated by two independent observers. Custom MATLAB scripts determined the mean choroidal thickness over a 3-mm subfoveal distance. We failed to acquire the line scan in one eye of one subject. Each scan was read by two observers and both measurements of choroid thickness were considered for statistical analysis from the remaining 45 images.